The types of question you will find in the test are of three kinds:
- Mnemonic. Some things you simply need to know, you cannot Google for them all the time. Take this as a training for Anatomy.
- Clinical cases. A patient has certain symptoms. Based on your biochemical knowledge, you are required to make an educated guess to the most likely disease affecting the individual.
- Biochemical scenarios. A certain situation in the cell is described, you must reason on the most likely consequence at the biochemical level. To answer these questions you must have a strong knowledge of the regulation of pathways, and their interplay.
Here are some sample questions, and a short discussion on how to get to the answer!
Q1. Which enzyme catalyzes the conversion of malate to oxaloacetate in the mitochondrion?
- Fumarate hydratase
- Malic enzyme
- Malate dehydrogenase
- Pyruvate carboxylase
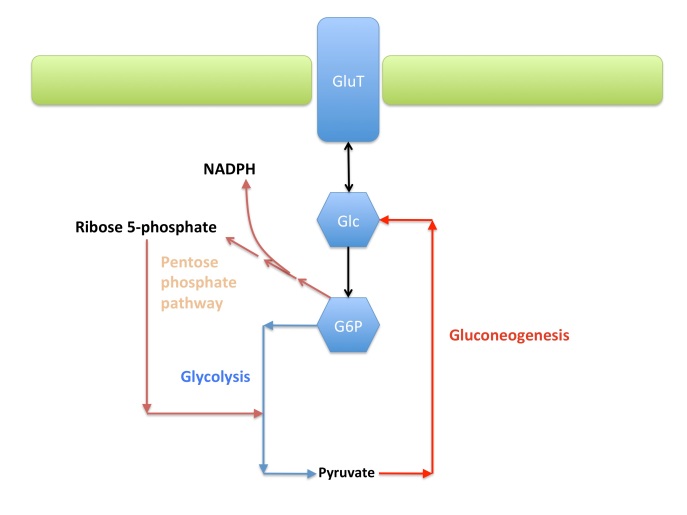
Ok, this is a typical mnemonic question. If you know that malate and oxaloacetate are intermediates in Krebs’ cycle and remember the name of the enzyme, you mark quickly the correct answer. But, the adrenaline of the exam may be confusing you. So, let’s work it out. Fumarate hydratase, as the name says, uses fumarate as a substrate, so we can discard that as well (in Krebs’ cycle fumarate gets hydrated to malate and is derived from succinate). Pyruvate carboxylase synthesizes oxaloacetate, but starting from pyruvate.You remember that malate and oxaloacetate traffic to the cytosol, and the malic enzyme is involved in their interconversion. Yes, but the question says “in the mitochondrion”. So it’s not the malic enzyme. So, the correct answer is Malate Dehydrogenase.
Q2. Which of the following metabolites can be uptaken and used by the brain as an energy source?
- Palmitic acid
- Acetyl-CoA
- beta-hydroxybutyrate
- gamma-aminobutirate (GABA)
Here you must remember that there is a barrier between the brain and the blood, so certain molecules cannot cross it. Both palmitic acid and acetyl-CoA are unable to reach the brain. However, the trick used in our organism is the production of ketone bodies (acetoacetate and beta-hydroxybutyrate) that can cross the BBB and be converted back to acetyl-CoA for energy production. GABA is a neurotransmitter, and its function is to deliver signals in the central nervous system by binding to specific receptors. Not exactly an energy-producing molecule.
Q3. A 10-year old boy is admitted in the emergency room because of sudden fainting and dispnea a one hours after a meal. At the objective exam he is jaundiced and negative for ketone bodies. Blood tests show a decrease in red blood cells. Pending other laboratory tests, which of the following is the most likely condition?
- Juvenile diabetes
- Phenylketonuria
- Haemolytic anemia
- Pancreatic cancer
Let’s look at the symptoms: the dispnea means he is not getting enough oxygen. Jaundice indicates an overload of the heme breakdown system. Negative for ketone bodies means the brain functions are fueled by glucose. Also, the symptoms appearing after a meal indicates that it is not a hypoglycemic effect.
We can discard diabetes, as the patients should be positive for ketone bodies (acetone breath). Phenylketonuria does not have anything to do with the situation, here. Pancreatic cancer may affect metabolism, and the typical first sign is jaundice. However, the anemia is not directly associated with PC. Thus, hemolytic anemia is the most likely condition. Since the symptoms appeared after a meal, you may inquire whether the patient had some oxidants-containing foods, suspecting favism.
Q4. The enzyme phosphofructokinase 1 (PFK1) catalyzes the committed step in ____ and (among other effectors) is activated by ____ and inhibited by _________.
- glycolysis, fructose 2,6 bisphosphate, citrate
- glycolysis, ATP, NADH
- gluconeogenesis, ATP, glucose 6-phosphate
- glycolysis, protein phosphatase 1, protein kinase A
The mnemonic aspect here is minimal. If you don’t remember that PFK1 works in glycolysis, you have hardly opened the book or read the material. Now, what activates and what inhibits? Since glycolysis is used to produce ATP, it makes no sense that ATP acts as an activator. PP1 and PKA have no established role in glycolysis. To be fair, the glucagon cascade leads to phosphorylation of PFK2, inactivating it and reducing the synthesis of F2,6-bP. But the question says “the enzyme PFK1”, and not “Glycolysis”. So, in case you did not know immediately, the answer is the first. Citrate signals a backlog in Krebs’ cycle, so you slow down glycolysis. F2,6-bP is an allosteric feedforward activator that also reduces the inhibition by ATP.
Q5. Which of the following organs can not use ketone bodies?
- Brain
- Liver
- Skeletal muscle
- Cardiac muscle
Ketone bodies are a soluble form of acetyl-CoA that can travel in the bloodstream. It is sensible that all organs may use them. Well, perhaps excluding the single organ that synthesizes them, otherwise they would not exit… Yes, it’s the liver, the site of synthesis.
Q6. The urea cycle is highly regulated to avoid ammonia toxicity. Under physiological conditions, the flux of metabolites in the cycle is strongly affected by_____.
- Bicarbonate
- Aspartate
- N-acetyl glutamate
- Glutamine
Bicarbonate and aspartate are reactants in the urea cycle. Under physiological conditions, these are not limiting, thus hardly affect the rate at wich the cycle proceeds. Glutamine is used to buffer the excess ammonia, and its concentrations do not affect directly the cycle. N-acetyl glutamate is synthesized when glutamate is abundant, and stimulates the oxidative deamination of glutamate by the NAD(P)+-dependent glutamate dehydrogenase. This enzyme provides the ammonium ion that enters the cycle, and determines the rate of the urea cycle.
Q7. How many ATP equivalents can be obtained from the complete aerobic oxidation of one butyric acid molecule?
- 2
- 10
- 32
- 128
Butyric acid is a C4:0 fatty acid. The question asks the maximum yield for its oxidation under aerobic conditions. Thus:
Butyric acid must first be linked to Coenzyme A. ATP is used in the process, yielding AMP + PPi, and PPi gets hydrolyzed to 2 inorganic phosphate molecules. So we use two ATP equivalents.
To completely oxidize C4 we need two rounds of mitochondiral beta oxidation. Each round of beta oxidation will yield 1 Acetyl-CoA, 1 NADH, and 1 FADH2. Thus, from beta oxidation 2 Ac-CoA, 2 NADH, 2 FADH2.
Each Acetyl-CoA will enter Krebs cycle, yielding 3 NADH, 1 FADH2, and 1 ATP (substrate level phosphorylation). Since we have 2 Acetyl-CoA molecules, we will get 6 NADH, 2 FADH2, and 2 ATP. So the total after beta oxidation and Krebs cycle is 8 NADH, 4 FADH2, and 2ATP. In oxidative phosphorylation, each NADH yields 3 ATP and each FADH2 2 ATP. So 8×3+4×2+2=34 ATP molecules. Note that the question explicitly asks for the ATP equivalents. We must subtract TWO ATPs, because we hydrolyzed two phosphoanhydridic bonds to promote the butyryl-CoA synthesis. So the answer is 32.
Q8. Which of the following best describes the process carried out by the aspartate-malate shuttle?
- It is essentially irreversible
- It transports NADH from the cytosol to the matrix
- During gluconeogenesis, malate moves to the cytosol
- It sustains glyceraldehyde 3-phosphate dehydrogenase activity during glycolysis
The shuttle is used to provide oxaloacetate during gluconeogenesis. Shuttles are by no means irreversible. NADH is not transported. GAPDH activity primarily relies on NAD+ regeneration through lactic acid fermentation.
Q10. Medium-chain acyl-CoA dehydrogenase (MCAD) is a mitochondrial enzyme that is essential for the beta oxidation of fatty acids. A patient with MCAD deficiency displays hypoglycemia because____
- The conversion of acetyl-CoA to pyruvate is inhibited
- MCAD activity is essential for the activity of fructose 1,6 bisphosphatase
- Medium chain fatty acids inhbit glycogen phosphorylase
- Beta oxidation cannot release acetyl-CoA to activate pyruvate carboxylase
When glucose levels drop, the liver attempts to provide the glucose via a) glycogen breakdown; b) gluconeogenesis. Hypoglycemia derives from the inability to perform such functions efficiently. Glycogen phosphorylase is not influenced by fatty acids, just by AMP, ATP, and glucose. Fructose 1,6 bisphosphatase is also not regulated by fatty acids. In humans, acetyl-CoA cannot be converted to pyruvate. Acetyl-CoA instead is a crucial activator of pyruvate carboxylase to its active form, the initial step of gluconeogenesis. So, a reduced MCAD activity limits the amounts of mitochondrial Acetyl-CoA, that in turn slows down gluconeogenesis and hence leads to hypoglycemia.
Q11. You are purifying a protein in the native state to perform structural and functional studies. At the present, your sample is contaminated by a second protein that has the same molecular weight, but differs in the isolectric point. Which chromatographic technique will give you the best chance of separating the two species?
- Affinity chromatography with the Ni-NTA resin
- Size-exclusion (gel filtration) chromatography
- Ion exchange
- SDS-PAGE
Affinity chromatography with Ni-NTA requires the protein of interest to have a polyhistidine tag, and this is not specified in the test. Size exclusion separates proteins based on size, and the two proteins have the same molecular weight, hence very similar sizes. SDS-PAGE denatures the proteins, so it is not a correct technique to purify proteins in the native state. Ion exchange (either cation or anion, depending on the pI of the two proteins) gives you the best chance for separation: you load the proteins onto the resin at a certain pH, and elute them with a salt gradient. The difference in pI will cause different affinities towards the resin, and they will detach from it at different elution volumes
Q12. How many base pairs are present in one complete turn of DNA double helix of the B form?
- 3.6
- 4.4
- 10
- 20
Ten bases, of course. Don’t be confused by 3.6, the number of residues in one turn of alpha helix in proteins, or 4.4 (pi helix, for those who went the extra length to read the table in the book). It is even correct in the hovering DNA…